
The ONLY non-crosslinked, non-avian
(bacterially fermented) Single-Injection
Hyaluronan (HA) Viscoelastic Hydrogel trusted for osteoarthritis (OA) knee pain relief
HYMOVIS® ONE is the ONLY non-crosslinked, single-injection, HA hydrogel viscosupplement available on the market in the US

Viscous (thick) gel-like sterile solution made from
highly purified chemically modified hyaluronan2
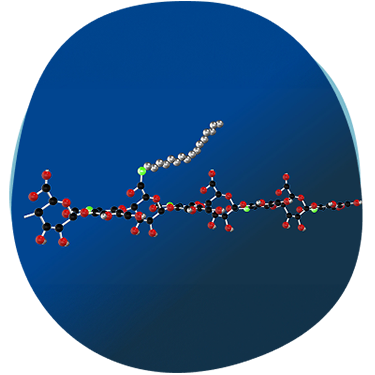
Minimally modified molecule with alkyl side chains, without any chemical cross-linking1
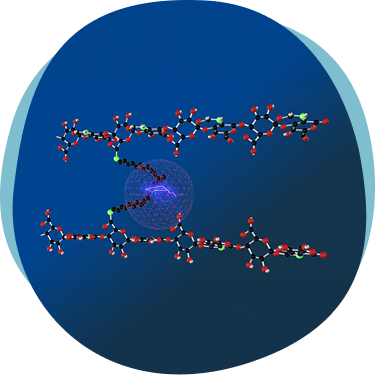
A network stabilized by reversable hydrophobic interactions, confering high viscoelasticity and
stability, resulting in an HA similar to the HA found in
the synovial fluid present in the human joint1,2
HYMOVIS® ONE is contraindicated in patients with known hypersensitivity (allergy) to hyaluronate preparations or gram-positive bacterial proteins. Do not administer
HYMOVIS® ONE to patients with infections or skin diseases in the area of the injection site or joint. The safety and effectiveness of HYMOVIS® ONE have not been established in
pregnant women, nursing mothers, or children, or for use in joints other than the knee, or for concomitant use with other intra-articular (IA) injections. The effectiveness of
repeat treatment cycles of HYMOVIS® ONE has not been established. No serious adverse events or pseudoseptic reactions were reported in the HYMOVIS® ONE clinical study.
The adverse events experienced and reported in the HYMOVIS® ONE clinical study were joint swelling, metatarsalgia, neck pain, and headache.
Rx Only. See package insert for full prescribing information, including indications, contraindications, adverse events, warnings, precautions, and side effects.
References:
1. Finelli I, et al. A new viscosupplement based on partially hydrophobic hyaluronic acid: A comparative study. Biorheology. 48 (2011): 263–275.
2. HYMOVIS® ONE Package Insert. Fidia Farmaceutici S.p.A., Abano Terme, Italy; 2025.