

SCIENTIFIC EVIDENCE
HYMOVIS® ONE is a true viscoelastic hydrogel – its unique molecular structure significantly
improved lubrication, reducing friction on cartilage in a preclinical analysis3,*
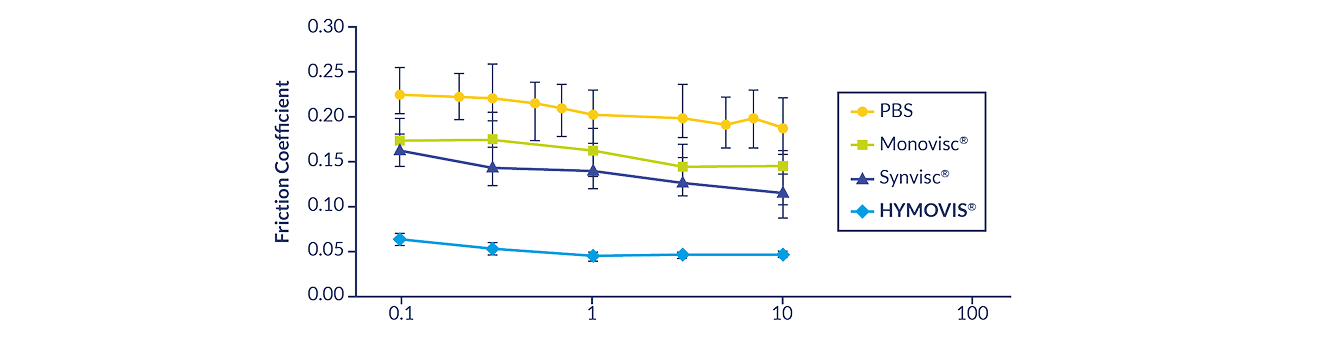
*Preclinical test data may not be indicative of human clinical outcomes.
Several hyaluronan (HA) viscosupplement products were assessed in this preclinical analysis. This graph demonstrates
preclinical comparison of selected single-injection HA viscosupplements.
The molecule of HYMOVIS® is the same as HYMOVIS® ONE. The molecule of Synvisc® is the same as Synvisc® One.
Third-party trademarks used herein are trademarks of their respective owners.
PBS = Phosphate buffered saline
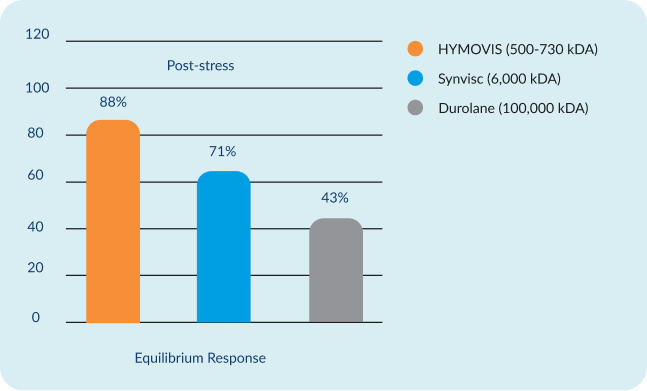
All 3 HAs started at a pre-stress baseline of 100% and had an instantaneous post-stress response of 29%*.
Following repeated stress, the structure of HYMOVIS® stabilizes due to the reversible hydrophobic interactions of the amide-containing alkyl side chains*.
Study design: Recovery of mechanical properties after a shock was investigated as a function
of time by oscillatory measurement after destruction of the gel network for each product: HYMOVIS®, Synvisc®, and Durolane®. The stress cycle was repeated 6 times.
*Preclinical test data may not be indicative of human clinical outcomes.
The molecule of HYMOVIS® is the same as HYMOVIS® ONE. The molecule of Synvisc® is the same as Synvisc® One.
Third-party trademarks used herein are trademarks of their respective owners.
HYMOVIS® ONE is contraindicated in patients with known hypersensitivity (allergy) to hyaluronate preparations or gram-positive bacterial proteins. Do not administer
HYMOVIS® ONE to patients with infections or skin diseases in the area of the injection site or joint. The safety and effectiveness of HYMOVIS® ONE have not been established in
pregnant women, nursing mothers, or children, or for use in joints other than the knee, or for concomitant use with other intra-articular (IA) injections. The effectiveness of
repeat treatment cycles of HYMOVIS® ONE has not been established. No serious adverse events or pseudoseptic reactions were reported in the HYMOVIS® ONE clinical study.
The adverse events experienced and reported in the HYMOVIS® ONE clinical study were joint swelling, metatarsalgia, neck pain, and headache.
Rx Only. See package insert for full prescribing information, including indications, contraindications, adverse events, warnings, precautions, and side effects.
References:
1. Finelli I, Chiessi E, Galesso D, Renier D, Paradossi G. A new viscosupplement based on partially hydrophobic hyaluronic acid: a comparative study. Biorheology. 2011;48(5):263-275.
3. Bonnevie ED, Galesso D, Secchieri C, Bonassar LJ (2019) Frictional characterization of injectable hyaluronic acids is more predictive of clinic outcomes than traditional rheological or viscoelastic characterization. PLoS ONE 14(5): e0216702. https://doi.org/10.1371/journal.pone.0216702