
Clinical Safety
& Efficacy
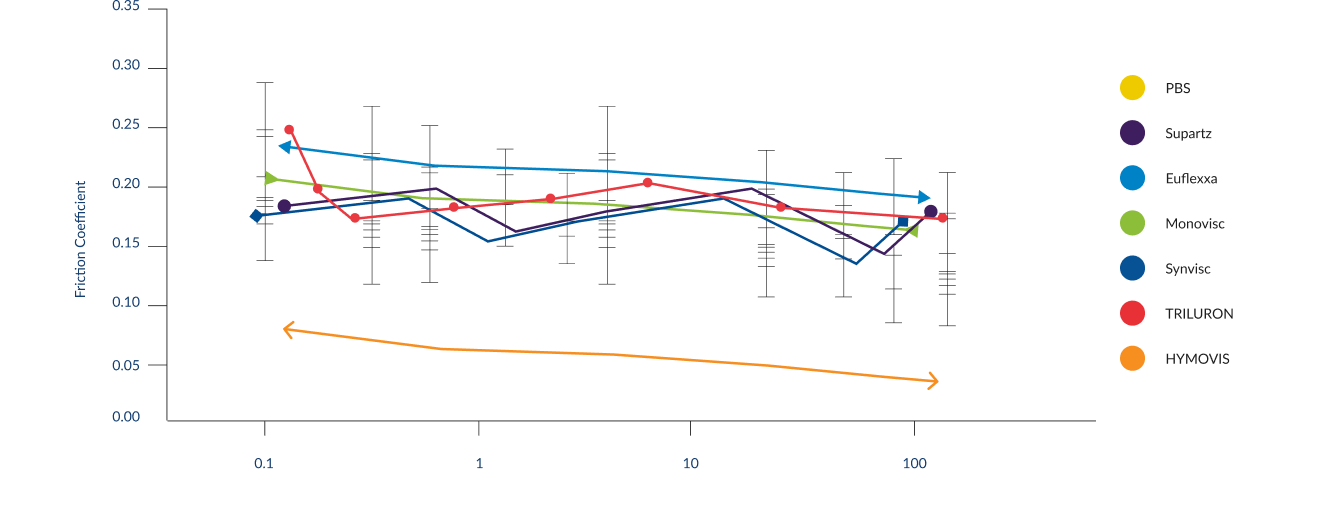
In a randomized controlled, single injection, active-comparator clinical study where
HYMOVIS® ONE was compared to a leading, commercially available, single-injection HA viscosupplement product
HYMOVIS® ONE delivered substantial, comparable pain relief and results for safety, with no pseudoseptic reactions and no serious adverse events, at 12 weeks (3 months) and 26 weeks (6 months).2,4
HYMOVIS® ONE also demonstrated significantly greater
improvement in Physical Function (WOMAC Function) at 6 months, favoring HYMOVIS® ONE compared to the other commercially available single-injection HA viscosupplement.2,4
HYMOVIS® ONE provides safe and effective, clinically meaningful benefits for reducing pain and improving joint function for patients with
osteoarthritis knee pain.2,4
HYMOVIS® ONE is indicated for the treatment of pain in osteoarthritis (OA) of the knee in patients who have failed to respond adequately to conservative non-pharmacologic therapy or simple analgesics (e.g., acetaminophen).
HYMOVIS® ONE is contraindicated in patients with known hypersensitivity (allergy) to hyaluronate preparations or gram-positive bacterial proteins. Do not administer
HYMOVIS® ONE to patients with infections or skin diseases in the area of the injection site or joint. The safety and effectiveness of HYMOVIS® ONE have not been established in pregnant women, nursing mothers, or children, or for use in joints other than the knee, or for concomitant use with other intra-articular (IA) injections. The effectiveness of repeat treatment cycles of HYMOVIS® ONE has not been established. No serious adverse events or pseudoseptic reactions were reported in the HYMOVIS® ONE clinical study.
The adverse events experienced and reported in the HYMOVIS® ONE clinical study were joint swelling, metatarsalgia, neck pain, and headache.
Rx Only. See package insert for full prescribing information, including indications, contraindications, adverse events, warnings, precautions, and side effects.
References:
2. HYMOVIS® ONE Package Insert. Fidia Farmaceutici S.p.A., Abano Terme, Italy; 2025.
4. HYMOVIS® ONE Summary of Safety and Effectiveness Data (SSED) P150010/S005.